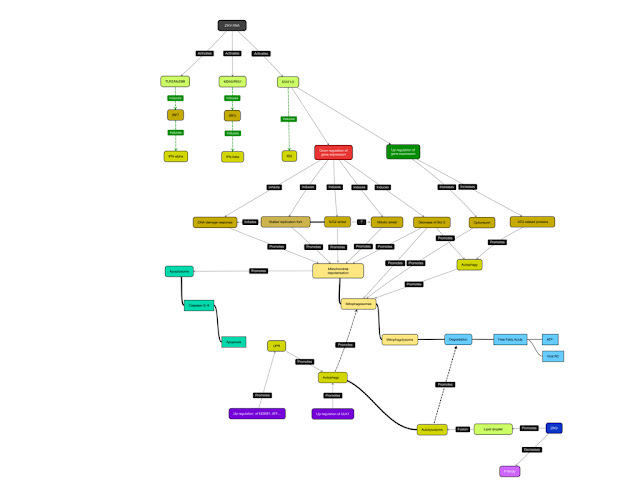
As
described in a previous post, Zika virus (ZIKV) infection of primary human skin
fibroblasts induces the upregulation of TLR3 mRNA as well as inducing the
expression of MDA-5 and RIG-1, components of the antiviral response induced not
only ZIKV but by RNA viruses in general. As a result of the activation of RIG-1
and MDA-5, the expression of both Interferon-α (IFN-α) and Interferon-β (IFN-β)
is increased. In mouse models such as A129, AG129 or Ifnar1 -/- mice that are
deficient for either the receptor for IFN-α or both for IFN-α and IFN-β, higher
viral loads are established in a number of tissues compared to wt mice,
suggesting that the induction of IFN-α and IFN-β by ZIKV limits viral
replication. Interestingly, in human neural progenitor cells (hNPC) infected
with ZIKV the expression of IFNAR-1 (the human equivalent of Ifnar-1) is
downregulated whereas the expression of the IFN-γ receptor (IFNGR-1) is
upregulated. The induction of the type I IFN response can however also
contribute to the severity of viral infections as shown for SARS-CoV in mice.
In infected mice, the delay of the induction of type I IFN by SARS-CoV
contributes to the accumulation of pathogenic inflammatory monocyte derived
macrophages, resulting in elevated levels cytokines and chemokines as well as
vascular leakage and the impairment of virus specific T cell responses, thus
contributing to decreased survival of infected mice compared to Ifnar-1 -/-
mice. Furthermore, blocking the IFNAR-1 receptor and deleting IFN-β in mice
promotes the clearance of lymphocytic choriomengitis virus (LCMV) in mice
whereas the activation of IFN-α inhibits early dissemination of LCMV. In
contrast to SARS-CoV and LCMV however, Ifnar1 -/-, A129 and AG129 mice show a
decreased survival rate, indicating that IFN-α is necessary for viral clearance
and survival of infected mice.
The
infection of hNPC with ZIKV MR766 induces the downregulation of a number of
genes promoting cell survival including those encoding for anti-apoptotic
proteins, cell cycle regulators, factors involved in the DNA damage response
pathway and autophagy as well inducing apoptosis that is preceded by an arrest
in G2/M phase of the cell cycle.
In
addition, human neurospheres and brain organoids infected with ZIKV MR766
exhibit extensive caspase-3 dependent cell death as early as 3 days p.i. which
is in agreement with previous results that showed that 56 hrs p.i. the
expression of Caspase-3 is upregulated, suggesting that in human neuronal cells
ZIKV induces Caspase-3 dependent apoptosis. None of these experiments however
determined the contributing factors, i.e. the pathway of apoptosis induction.
The
infection of human lung epithelial A549 cells with ZIKV PF/25013/18 results in
effective viral replication as measured by immunofluorescence for the viral E
protein and dsRNA, flow cytometry for E protein positive cells, and determination
of viral titres that showed maximum titres within 48 hrs p.i. concomitant with
the induction of PARP cleavage and decreased cell viability. RT-PCR analysis of
ZIKV infected cells revealed that ZIKV infection induces a 200-fold increase of
both IFIT-1 (ISG56) and IFIT-2 (ISG54), both of which are regulated by IRF-3
and IRF-7 as well as STAT-1/-2 (in addition to A549 cells, ZIKV also
upregulates IFIT-2 and IRF-7 expression in hNPC). Since IFIT-2 induces the
depolarization of mitochondria and thus contributes to the induction of
Caspase-3 and -9 dependent apoptosis, it might be possible that the formation of
the complex consisting of IFIT-1 and IFIT-2 is induced by ZIKV and induces
apoptosis in a Bak and Bax dependent manner. Unfortunately, the current data do
not contain experiments using either Bak -/- Bax -/- cells nor cells that are
deficient for either IFIT-1/-2 or cells treated with siRNA targeting Bak, Bax,
IFIT-1 or IFIT-2. Downstream of IFIT-1 and IFIT2, the infection of A549 cells
with ZIKV triggers the production of mitochondrial reactive oxygen species
(ROS) as detected by MitoSOX without increasing cytoplasmic ROS levels similar
to cells infected with DENV by increasing the expression of mitochondrial SOD2
although in the case of DENV infected cells cytoplasmic ROS levels are
increased as the result of DENV induced activation of the ER stress response.
If the downregulation of RecQL4 expression in ZIKV infected cells contributes
to the activation of SOD2 is a contributing factor is not known, but possible. Alternatively,
ZIKV proteins localizing to the ER might induce the ER stress response and thus
induce the expression of mitochondrial ROS independent of IFIT-1/-2.
 |
| Figure: Changes of gene expression in hNPC infected with ZIKV compared to Mock infected cells; RECQL4, IFNAR-1, IRF-7, IFIT-2 |
Also
it remains to be seen if the expression of IFN-β in ZIKV infected cells can be
induced via the relocalisation of STING to perinuclear punctae.
The
infection of primary human skin fibroblasts with ZIKV induces the expression of
pro-inflammatory cytokines which can also be detected in patients infected with
ZIKV. Accordingly, the infection of A549 cells induces the expression of IL-1β,
IL-6 and MCP-1 at 24-48 hrs p.i.. IFN-β expression and secretion can be
detected at early times post infection (12 hrs p.i., increasing between 18-24
hrs p.i.), suggesting that the induction of IFN-β increases the expression of
both IFIT-1 and IFIT-2 contributing to the induction of apoptosis.
Interestingly, pretreatment of A549 cells with IFN-β not only reduces viral
titres but also reduces caspase-3 activity suggesting that other factors than
the IFIT-1/IFIT-2 complex trigger apoptosis. Since IFN-β not only triggers an
antiviral response and apoptosis but also autophagy, pretreatment of A549 cells
might induce the formation of autophagosomes that not only promotes the
degradation of viral particles upon entry but also promotes the degradation of
viral RNA in a process called RNautophagy. Further studies are however needed
to verify and explore this pathway. Since the infection of mouse embryonic
fibroblasts (MEF) with West Nile Virus (WNV) or Japanese Encephalitis Virus
(JEV) activates the inflammasome, pretreatment of A549 cells might prime cells
to MEFV dependent inactivation of the inflammasome and thus apoptosis following
inflammasome activation by ZIKV.
 |
| Figure: Induction of SOD2: dependent on downregulation of RECQL4 and/or induction of ER stress response |
In
conclusion, the infection of A549 cells with ZIKV results in productive
infection and triggers an antiviral response that includes the expression of
chemokines, IFN-β, and IFIT-1 as well as IFIT-2, similar to observations from
ZIKV patients or ZIKV infected hNPC. Similar to hNPC, neurospheres, and brain
organoids, ZIKV triggers caspase dependent apoptosis probably in a IFIT-1/-2
independent manner, although further experiments are warranted. Pretreatment
and treatment up to 2 hrs p.i. of A549 cells with IFN-β results in decreased
viral titres and decreased caspase-3 activation suggesting that IFN-β has a
pro-survival rather than a pro-apoptotic role in ZIKV infection, a notion that
is supported by observations from existing mouse models. The increase of
mitochondrial ROS by ZIKV might induce DNA damage that due to the potential
inhibition of the DNA damage response by ZIKV might induce cell cycle arrest
and subsequent apoptosis as well MAVS/RIG1 mediated induction of IFN expression
and/or MAPK/NFκ-B mediated increase of chemokines.
Further reading
Chan JF, Choi GK, Yip CC, Cheng VC, & Yuen KY (2016). Zika fever and congenital Zika syndrome: An unexpected emerging arboviral disease. The Journal of infection PMID: 26940504
Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, & Perlman S (2016). Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell host & microbe, 19 (2), 181-93 PMID: 26867177
Ng CT, Sullivan BM, Teijaro JR, Lee AM, Welch M, Rice S, Sheehan KC, Schreiber RD, & Oldstone MB (2015). Blockade of interferon Beta, but not interferon alpha, signaling controls persistent viral infection. Cell host & microbe, 17 (5), 653-61 PMID: 25974304
Frumence E, Roche M, Krejbich-Trotot P, El-Kalamouni C, Nativel B, Rondeau P, Missé D, Gadea G, Viranaicken W, & Desprès P (2016). The South Pacific epidemic strain of Zika virus replicates efficiently in human epithelial A549 cells leading to IFN-β production and apoptosis induction. Virology, 493, 217-226 PMID: 27060565
Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, Christian KM, Didier RA, Jin P, Song H, & Ming GL (2016). Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell stem cell PMID: 26952870
Faria NR, Azevedo RD, Kraemer MU, Souza R, Cunha MS, Hill SC, Thézé J, Bonsall MB, Bowden TA, Rissanen I, Rocco IM, Nogueira JS, Maeda AY, Vasami FG, Macedo FL, Suzuki A, Rodrigues SG, Cruz AC, Nunes BT, Medeiros DB, Rodrigues DS, Nunes Queiroz AL, Silva EV, Henriques DF, Travassos da Rosa ES, de Oliveira CS, Martins LC, Vasconcelos HB, Casseb LM, Simith DB, Messina JP, Abade L, Lourenço J, Alcantara LC, Lima MM, Giovanetti M, Hay SI, de Oliveira RS, Lemos PD, Oliveira LF, de Lima CP, da Silva SP, Vasconcelos JM, Franco L, Cardoso JF, Vianez-Júnior JL, Mir D, Bello G, Delatorre E, Khan K, Creatore M, Coelho GE, de Oliveira WK, Tesh R, Pybus OG, Nunes MR, & Vasconcelos PF (2016). Zika virus in the Americas: Early epidemiological and genetic findings. Science (New York, N.Y.) PMID: 27013429
Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Desprès P, Amara A, Yssel H, & Missé D (2015). Biology of Zika Virus Infection in Human Skin Cells. Journal of virology, 89 (17), 8880-96 PMID: 26085147
Lazear, H., Govero, J., Smith, A., Platt, D., Fernandez, E., Miner, J., & Diamond, M. (2016). A Mouse Model of Zika Virus Pathogenesis Cell Host & Microbe DOI: 10.1016/j.chom.2016.03.010
Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, & Weaver SC (2016). Characterization of a Novel Murine Model to Study Zika Virus. The American journal of tropical medicine and hygiene PMID: 27022155
Stawowczyk, M., Scoy, S., Prasanna Kumar, K., & Reich, N. (2011). CS18-6. Interferon stimulated gene 54 (ISG54) promotes apoptosis Cytokine, 56 (1) DOI: 10.1016/j.cyto.2011.07.427
Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, & Rehen SK (2016). Zika virus impairs growth in human neurospheres and brain organoids. Science (New York, N.Y.) PMID: 27064148
Kumari J, Hussain M, De S, Chandra S, Modi P, Tikoo S, Singh A, Sagar C, Sepuri NB, & Sengupta S (2016). Mitochondrial functions of RECQL4 are required for the prevention of aerobic glycolysis-dependent cell invasion. Journal of cell science, 129 (7), 1312-8 PMID: 26906415
Andersen J, VanScoy S, Cheng TF, Gomez D, & Reich NC (2008). IRF-3-dependent and augmented target genes during viral infection. Genes and immunity, 9 (2), 168-75 PMID: 18094709
Qiao C, Lu N, Zhou Y, Ni T, Dai Y, Li Z, Guo Q, & Wei L (2016). Oroxylin a modulates mitochondrial function and apoptosis in human colon cancer cells by inducing mitochondrial translocation of wild-type p53. Oncotarget PMID: 26958937
Cao Z, Zhou Y, Zhu S, Feng J, Chen X, Liu S, Peng N, Yang X, Xu G, & Zhu Y (2016). Pyruvate Carboxylase Activates the RIG-I-like Receptor-Mediated Antiviral Immune Response by Targeting the MAVS signalosome. Scientific reports, 6 PMID: 26906558
West, A., Shadel, G., & Ghosh, S. (2011). Mitochondria in innate immune responses Nature Reviews Immunology, 11 (6), 389-402 DOI: 10.1038/nri2975
Ambjørn M, Ejlerskov P, Liu Y, Lees M, Jäättelä M, & Issazadeh-Navikas S (2013). IFNB1/interferon-β-induced autophagy in MCF-7 breast cancer cells counteracts its proapoptotic function. Autophagy, 9 (3), 287-302 PMID: 23221969
Aizawa S, Fujiwara Y, Contu VR, Hase K, Takahashi M, Kikuchi H, Kabuta C, Wada K, & Kabuta T (2016). Lysosomal putative RNA transporter SIDT2 mediates direct uptake of RNA by lysosomes. Autophagy, 12 (3), 565-78 PMID: 27046251
Kimura T, Jain A, Choi SW, Mandell MA, Johansen T, & Deretic V (2016). TRIM-Directed Selective Autophagy Regulates Immune Activation. Autophagy PMID: 26983397
Suthar, M., Aguirre, S., & Fernandez-Sesma, A. (2013). Innate Immune Sensing of Flaviviruses PLoS Pathogens, 9 (9) DOI: 10.1371/journal.ppat.1003541
Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, & Kaufman RJ (2013). ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nature cell biology, 15 (5), 481-90 PMID: 23624402
Wei H, Kim SJ, Zhang Z, Tsai PC, Wisniewski KE, & Mukherjee AB (2008). ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Human molecular genetics, 17 (4), 469-77 PMID: 17989065
Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, & Kaufman RJ (2013). ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nature cell biology, 15 (5), 481-90 PMID: 23624402
Wei H, Kim SJ, Zhang Z, Tsai PC, Wisniewski KE, & Mukherjee AB (2008). ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Human molecular genetics, 17 (4), 469-77 PMID: 17989065