Zika
Virus (ZIKV) is a emerging positive strand RNA virus that belongs to the genus
of Flaviviridae. Human infections are
mostly asymptomatic although a causative link to microcephaly and Guillan-Barre
Syndrome (GBS) have been proposed.
So
far however no reliable animal model to study ZIKV associated disease has been
identified but in order to test a potential vaccine and order to gain a better
understanding of ZIKV pathogenesis animal models are being developed. Similar to
DENV, these models may involve immunocompetent and humanized mice as well
nonhuman primates.
Here
recent advances to establish a mouse model are presented.
ZIKV in mice
In
the original mouse model, ZIKV was first isolated from Swiss albino mice
intracerebrallly injected with the original ZIKV 766 strain that was isolated
from the sentinel rhesus monkey as well as virus suspensions obtained from
infected Aedes Africanus mosquitoes.
Following the 16 passages of ZIKV strain 766 in mice, 100% mortality can be
observed and subsequent infections of mice with (mouse adapted) ZIKV 766 lead
to a mean survival time of 10.6 days with an incubation period of 6 days.
Both
the infection of mice with ZIKV 758 isolated from rhesus monkeys and the E/1
strain isolated from infected A.
Africanus resulted in 100% mortality at passage 16 and 15 indicating that
the intracerebral injection of ZIKV suspensions in and subsequent adaption of
rhesus and mosquitoe derived ZIKV to mice results in sickness (indicated by
roughness of the fur and inactivity), paralysis (motor weakness and paralysis
of limbs) and death within 24 to 48 hrs p.i. .
 |
| Figure: ZIKV in Swiss albino mice: number of healthy, sick and paralysed mice |
 |
| Figure: ZIKV in Swiss albino mice: number of dead mice |
Subsequent
intracerebral (i.c.) infection of newborn and 5 week old mice with ZIKV MP1751
strain showed that ZIKV replicates in both neurons and astroglial cells as well
as extensive cell death in the hippocampus and cortex of infected mice,
suggesting that apoptosis or necrosis of brain cells might be the cause of
paralysis. Recently published data obtained from human cortical neuron
progenitor cells infected with ZIKV 766 indicate that infected neuronal cell
indeed undergo apoptosis probably by ZIKV induced downregulation of
anti-apoptotic genes, thus explaining the loss of neuronal cells in ZIKV (intercelebrally;
i.c.) infected mice. The initial
experiments also revealed that mice infected intraperitoneally (i.p.) at 7 days
of age exhibited a higher LD50 value than those 14 days or older at
time of infection although mice older than 6 weeks are slightly less
susceptible than younger mice to i.c. infection.
Interestingly, recent data indicate that the infection of 7 days old mice intraperitoneally infected with ZIKV Dakar 41519 reduces viability by 33% compared to mice infected subcutaneously (s.c.), suggesting that the route of infection as well as the age at time of infection indeed effects the survival rate.
 |
| Figure: LD50 values are influenced by age of mice at time of infection |
Interestingly, recent data indicate that the infection of 7 days old mice intraperitoneally infected with ZIKV Dakar 41519 reduces viability by 33% compared to mice infected subcutaneously (s.c.), suggesting that the route of infection as well as the age at time of infection indeed effects the survival rate.
Initially,
ZIKV was not detected in any other organs following infection i.c., but in more
recent studies in A129 and AG129 mice infected either i.p. or intradermal
(i.d.) with ZIKV FSS13025 (Asian lineage) showed high viral titres in the
kidney, spleen, testes, and brain of infected mice suggesting that the route of
infection might not only determine the survival rate but also sites or viral
replication. Comparing these results with those obtained originally using Swiss
albino mice suggest that ZIKV infection in mice induces the antiviral
Interferon response since A129 mice are deficient for both Interferon-α and –β
(IFN-α /-β) whereas AG129 are deficient not only for IFN-α /-β but also for
IFN-γ. The notion that ZIKV induces
the interferon response is supported by recently published studies in primary
skin fibroblasts and human placenta cells as well as in triple knockout mice.
ZIKV: induction
of the Interferon response
Infection
of primary human skin fibroblasts with low passage ZIKV strain derived from a
viremic patient in Tahiti/French Polynesia, PF-25013-18, results in the
activation of the innate immune response namely the induction of Pattern
Recognition Receptors (PRRs) by the viral RNA. As indicated by analysing the
expression of genes using qRT-PCR, the expression of TLR3, RIG-1/DDX58 and
MDA5/IFIH1 as well as CXCL10, Interleukin-1β, AIM2 is upregulated whereas the
expression of others such as TLR7, TLR8, or TNF is downregulated as soon as 6
hrs p.i.. Activation of TLR3, RIG1 and MDA5 expression increases also the
expression of Interferon stimulated genes (ISG) including OAS2, ISG15 and MX1,
suggesting that ZIKV RNA activates PRRs dependent downstream signaling pathways
by binding to TLR3, MDA5 and RIG-1 which subsequently stimulate the expression
of ISG via upregulation of IRF7 expression. Since IRF7 is a transcription
factor for type I interferons, the infection of primary skin cells with ZIKV
induces an antiviral response that decreases viral titres.
Since
human placental trophoblasts (PHT) are resistant to infection with both ZIKV
FSS13025, a strain isolated in Cambodia and the original ZIKV MR766 strain,
ZIKV might induce an antiviral response similar to those observed in primary
human skin cells. Indeed, ZIKV infected PHT cells exhibit a high level of type
III Interferon, in particular Interferon-λ1 (IFNλ1) which is constitutively
expressed by PHT cells. Similar to primary skin fibroblast cells the expression
of OAS2, TLR3 and MX2 is unregulated when compared to human choriocarcinoma
JEG-3 that support ZIKV replication, suggesting that ZIKV induces identical
antiviral signaling pathways in both PHT and skin cells. Furthermore,
conditioned media from infected PHT cells inhibits the replication of ZIKV in
JEG-3 cells by inducing the expression of ISG, suggesting that PHT inhibit ZIKV
replication by inducing the expression in a autocrine and paracrine manner
although further work is needed verify the hypothesis.
The
finding that ZIKV replication in PHT might be inhibited by IFNλ1 causes however some
problems in the utilization of mice as a model to investigate the potential relationship
between ZIKV and microcephaly in neonates and/or other embryonal development
defects since not only is there a difference in the morphology of the placenta
but also in the expression of IFNλ subtypes; mice only express IFNλ2 and IFNλ3
whereas PHT only low levels of IFNλ2 and no IFNλ3.
The importance of the role
of the interferon response in controlling the replication of ZIKV and
preventing the onset of neurological symptoms resembling those observed in
infected humans is further strengthened by
studies using mice deficient for components of the interferon and comparing
them to fully immunocompetent mice. These experiments differ from those
conducted in Swiss albino mice described above in so far, as this model allows not only the use of wt ZIKV strains or ZIKV strains
that exhibit only low passage number in Vero or C6/36 mosquitoe cells but also prevents
the need of infection via intracelebral injection, thus avoiding the need for the
virus to cross the blood-brain barrier.
Similar to Swiss albino
mice injected i.c. with ZIKV 766, the injection of immunocompetent C57BL/6 5 to
6 week old mice with ZIKV MR766 or H/PF/2013 either s.c. or intravenous (i.v.)
does not induce the development of any neurological symptoms up to 10 days p.i.
nor weight loss. As mentioned above, the infection of immunocompetent mice 7
days of age with ZIKV Dakar however decreases survival by 33% at day 30 p.i. ,
suggesting that either age or route of infection might play a role in the
severity of the disease or alternatively that ZIKV strains of the African
lineage are more virulent than those of the Asian lineage.
In
order to determine if components of the interferon pathway prevent the
onset of symptoms and/or death of infected mice after the infection with ZIKV,
4-6 week old mice deficient for the transcription factors IRF3, IRF5 and IRF7
as well as deficient for the Ubiquitin-like protein ISG15 were
infected with ZIKV MR766 or ZIKV H/PF/2013 i.v. or s.c. and monitored for signs
of infection for 10 days p.i. . In contrast to the single knockout mice, triple
knockout (TKO) IRF3 -/- IRF5 -/- IRF7 -/- mice exhibited symptoms such as
paralysis as early as 3 days p.i. regardless of the infection route. In
addition, up to 100% of infected TKO mice were dead 10 days p.i. whereas single
knockouts survived. In a similar way, single Ifnar-1 (Interferon-α receptor)
knockout mice infected with ZIKV Dakar 41519, ZIKV MR766 or ZIKV H/PF/2013
exhibited 100% mortality 10 days p.i. whereas infected single MAVS -/- knockout
mice did exhibit any mortality. Blocking the the IFNAR-1 receptor and thus IFN-
α/β using a monoclonal antibody one day prior ZIKV infection renders wt mice
susceptible to ZIKV which is similar to results described for West Nile Virus
(WNV), although blockage of IFNAR-1 does not increase mortality but induces
higher viral titres. Interestingly older Ifnar1 -/- mice exhibit a higher
survivial rate with 40-80% of infected mice despite weight loss; whether viral
loads are lower as well has not been investigated.
Similar to ZIKV infected A129 and AG129 mice, in Ifnar1 -/- mice the
tissues with the highest viral load are spleen, testes, kidneys, spinal cord,
brain and liver thus confirming previous reports that ZIKV is a
neurotropic virus. In addition, the brain and testes of surviving Ifnar1 -/-
exhibit high viral RNA levels up to 28
days p.i., a finding that might explain the occurrence of neurological symptoms as well as horizontal transmission of ZIKV to previously uninfected women. From the present epidemiological data however it is not clear if ZIKV induces encephalitis at a similar rate than those observed in patients infected with DENV or WNV.
 |
| Figure: Survival rates of immunocompetent and immunodeficient mice |
Taken together with the
gene expression data obtained from JEG-3 and PHT cells, these results suggest
that the interferon response does protect not only placenta derived cells but
also mice from ZIKV induced death similar to WNV. Further data however are
needed if ZIKV neurovirulence in the embryo or foetus dependent on the
resistance of ZIKV to interferon induced signalling or due to the induction of
cell cycle arrest and subsequent apoptosis or neural progenitor cells.
ZIKV in rhesus macaque monkeys
In
general monkeys infected with ZIKV do not exhibit any or only mild clinical
signs of infections although virus can be detected as early as 3 days p.i. as
suggested by historic data.
In
order to assess the the infectivity of the original ZIKV MR766 isolate from Africa,
experiments are currently conducted at the University of Wisconsin-Madison that
determine the load of viral RNA in the blood, urine, saliva and cerebrospinal
fluid (CSF)
of rhesus macaque monkeys infected with different
doses of ZIKV. In both experiments, 2 out of 3 animals exhibit maximum load
viral RNA in plasma at 3-5 days p.i. and in the urine at 7-9 days p.i. .
 |
| Figure: Load of viral RNA in rhesus macaque infected with ZIKV |
In addition, similar studies are currently performed to determine if the infection of monkeys with ZIKV induces the formation of malformations in the brain during the foetal development, but as the time of writing these studies have not concluded.
In conclusion, the infection of immunocompetent
mice with ZIKV is not lethal nor do those mice present themselves with clinical
symptoms such as paralysis. Mice deficient for interferon dependent antiviral
signaling however succumb to infection 10 days p.i., indicating that ZIKV is
not resistant to interferon signaling per se. In addition, in primary human placental trophoblasts ZIKV replication is
inhibited by IFNλ1, whereas previous studies suggest that human NPC and human
stem cells are supporting ZIKV replication. This suggests a model where ZIKV
needs to breach the placental-foetal barrier in order to infect embryonal cells
without infecting placental cells, suggesting that infected maternal cells need
to cross the barrier.
The activation of the interferon response by ZIKV
RNA however might not only induce antiviral signaling but also induce the up-
and downregulation of gene expression via inducing STAT1 and 2 mediated
signaling. In this model, STAT-1/-2 regulates the expression of genes encoding
for proteins regulating diverse processes as DNA repair, cell cycle control and
autophagy, thus contributing to cell death observed in infected hNPC as
discussed before.
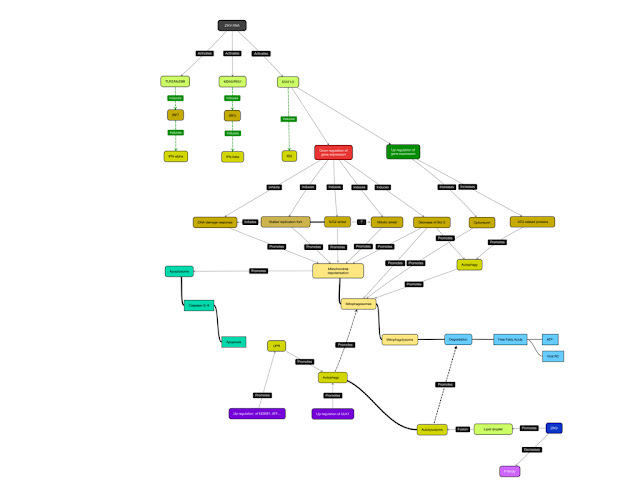 |
| Figure: ZIKV RNA induces not only antiviral signalling but also regulates gene expression |
Further reading
Chan KW, Watanabe S, Kavishna R, Alonso S, & Vasudevan SG (2015). Animal models for studying dengue pathogenesis and therapy. Antiviral research, 123, 5-14 PMID: 26304704
DICK GW (1952). Zika virus. II. Pathogenicity and physical properties. Transactions of the Royal Society of Tropical Medicine and Hygiene, 46 (5), 521-34 PMID: 12995441
Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, & Weaver SC (2016). Characterization of a Novel Murine Model to Study Zika Virus. The American journal of tropical medicine and hygiene PMID: 27022155
Lazear, H., Govero, J., Smith, A., Platt, D., Fernandez, E., Miner, J., & Diamond, M. (2016). A Mouse Model of Zika Virus Pathogenesis Cell Host & Microbe DOI: 10.1016/j.chom.2016.03.010
Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Desprès P, Amara A, Yssel H, & Missé D (2015). Biology of Zika Virus Infection in Human Skin Cells. Journal of virology, 89 (17), 8880-96 PMID: 26085147
Bell TM, Field EJ, & Narang HK (1971). Zika virus infection of the central nervous system of mice. Archiv fur die gesamte Virusforschung, 35 (2), 183-93 PMID: 5002906
Bell TM, Field EJ, & Narang HK (1971). Zika virus infection of the central nervous system of mice. Archiv fur die gesamte Virusforschung, 35 (2), 183-93 PMID: 5002906
Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, Christian KM, Didier RA, Jin P, Song H, & Ming GL (2016). Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell stem cell PMID: 26952870
Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, & Kriegstein AR (2016). Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell stem cell PMID: 27038591
Atkins GJ, & Sheahan BJ (2016). Molecular Determinants of Alphavirus Neuropathogenesis in Mice. The Journal of general virology PMID: 27028153
Liang J, Piao Y, Henry V, Tiao N, & de Groot JF (2015). Interferon-regulatory factor-1 (IRF1) regulates bevacizumab induced autophagy. Oncotarget, 6 (31), 31479-92 PMID: 26362401
Lefèvre F, Guillomot M, D'Andréa S, Battegay S, & La Bonnardière C (1998). Interferon-delta: the first member of a novel type I interferon family. Biochimie, 80 (8-9), 779-88 PMID: 9865499
Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, Gale M Jr, Klein RS, & Diamond MS (2015). Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Science translational medicine, 7 (284) PMID: 25904743
Rowland, A., Washington, C., Sheffield, J., Pardo-Villamizar, C., & Segars, J. (2016). Zika virus infection in semen: a call to action and research Journal of Assisted Reproduction and Genetics, 33 (4), 435-437 DOI: 10.1007/s10815-016-0684-6



No comments:
Post a Comment